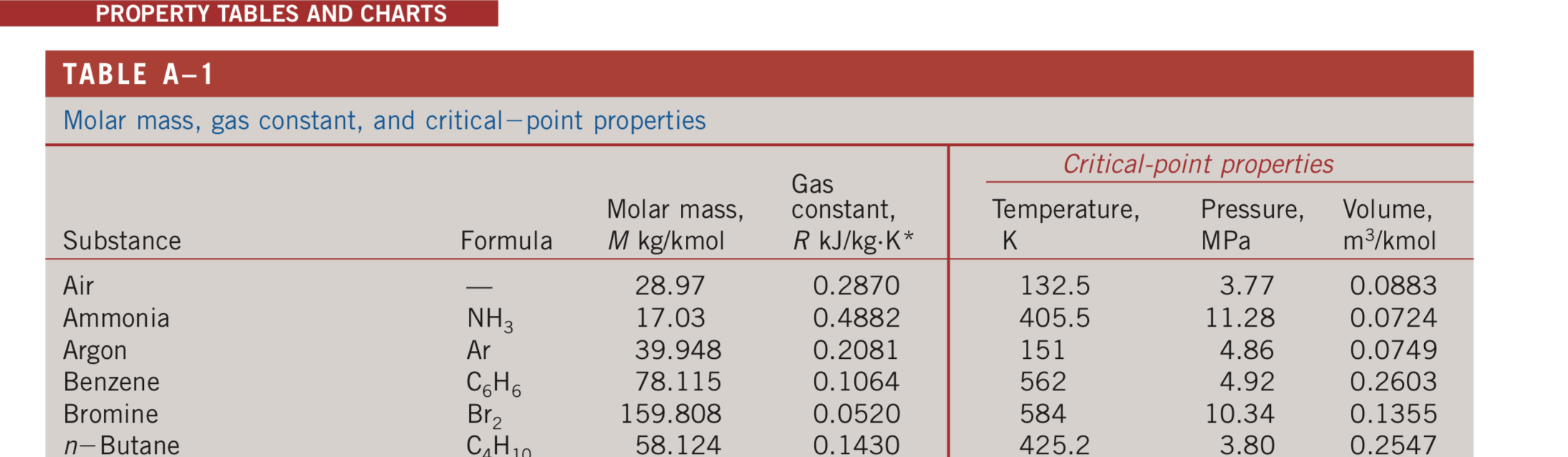
Polynomial constants for specific heat at constant pressure c p (J/kg.K). | Download Scientific Diagram

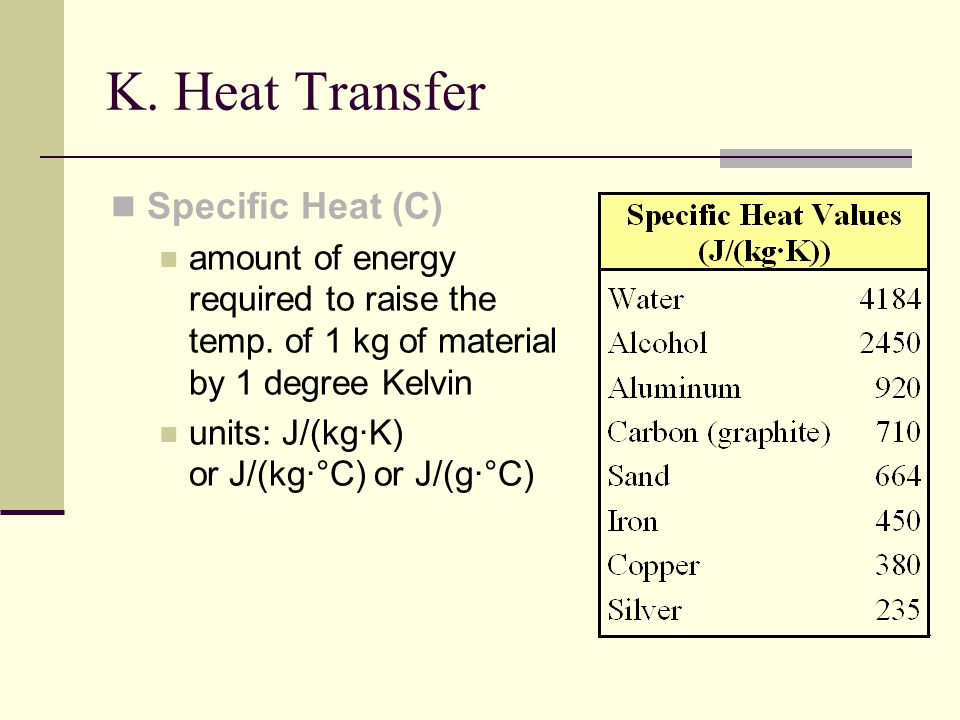
The specific heat of air at constant pressure is 1.05kj//kg K and the specific heat of air at - YouTube

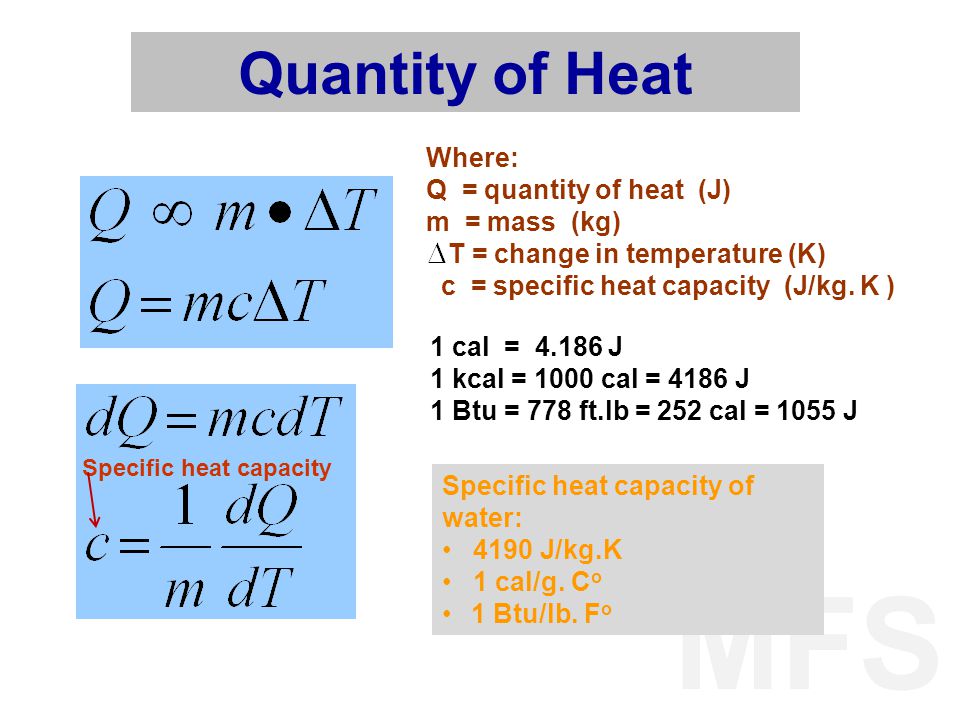
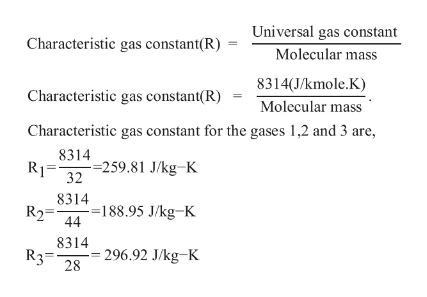
The temperature of 3 kg of nitrogen is raised from 10 ^o C to 100 ^o C. Compute the difference in work done if heating were at constant pressure and at constant

For a gas the difference between the two specific heats is 4150 J/kg - K . What is the specific heat at constant volume of gas if the ratio of specific heats is 1.4 ?
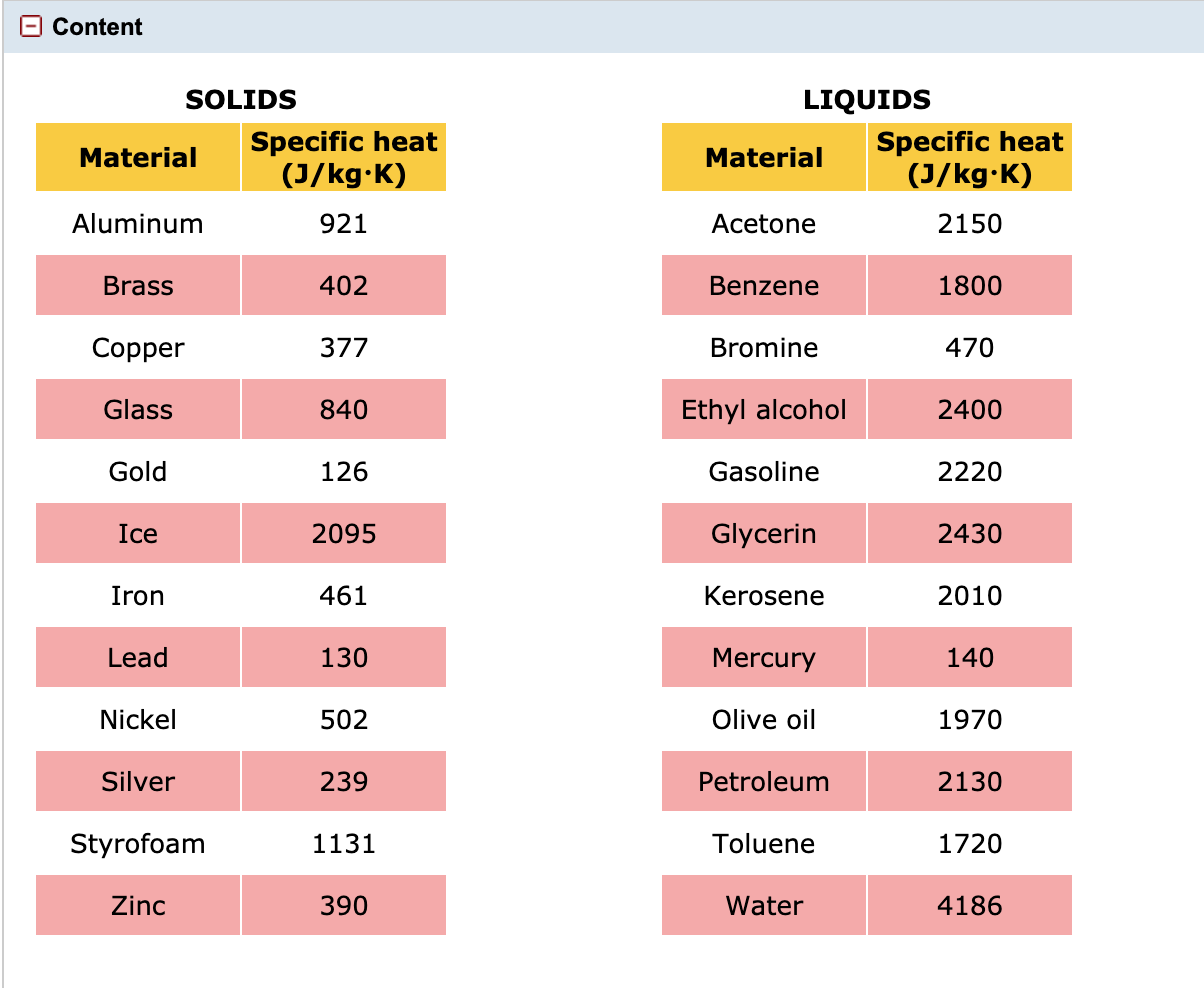
![Specific heat capacity C p [J/(kg·K)] of small samples (E, F, and G) at... | Download Scientific Diagram Specific heat capacity C p [J/(kg·K)] of small samples (E, F, and G) at... | Download Scientific Diagram](https://www.researchgate.net/profile/Radoslaw-Wach/publication/256115072/figure/tbl1/AS:755605098729495@1557161702871/Specific-heat-capacity-C-p-J-kgK-of-small-samples-E-F-and-G-at-various.png)
Specific heat capacity C p [J/(kg·K)] of small samples (E, F, and G) at... | Download Scientific Diagram

Calculate the heat required to convert 3 kg of ice at - 12^o C kept in a calorimeter to steam at 100^o at atmospheric pressure. (Given: specific heat of ice = 2.100 ×