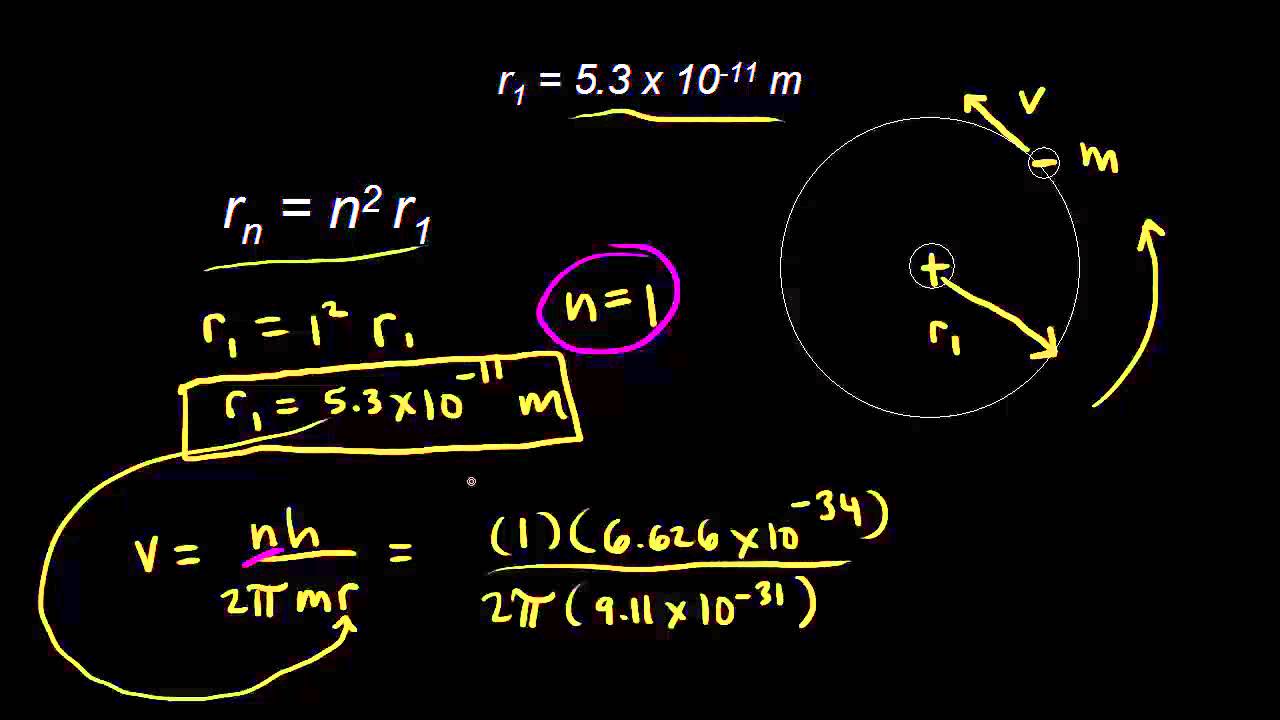different between nth and `(n + 1) th` Bohr's radius of hydrogen atom is equal to `(n = 1) - YouTube
Derive a formula for radius of the stable orbit of hydrogen atom on the basis of Bohr model. Prove that in hydrogen - Sarthaks eConnect | Largest Online Education Community

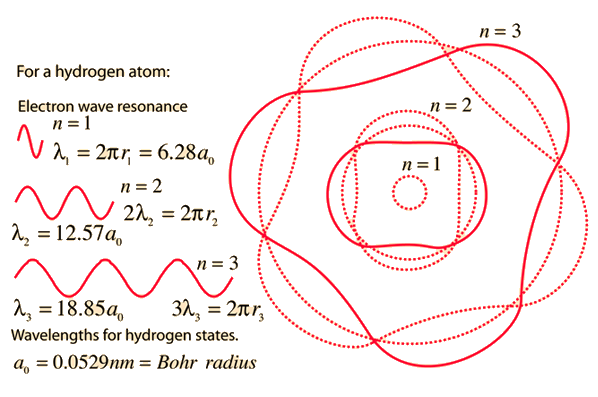
MathType on Twitter: "The most probable distance between the nucleus and the electron in a hydrogen atom in its ground state is given by the Bohr Radius. This physical constant is named

OpenStax College Physics Solution, Chapter 30, Problem 13 (Problems & Exercises) | OpenStax College Physics Answers

In the Bohr's model of hydrogen - like atom the force between the nucleus and the electron is modified as F = e^24piε0 ( 1r^2 + betar^3 ) , where beta is

Write an expression for Bohr's radius in hydrogen atom. | 12 | ATOMS AND NUCLEI | PHYSICS | PRA... - YouTube

Determining Radius Of An Orbit Using Bohr's Equation | 1st year Chemistry | swap education portal - YouTube

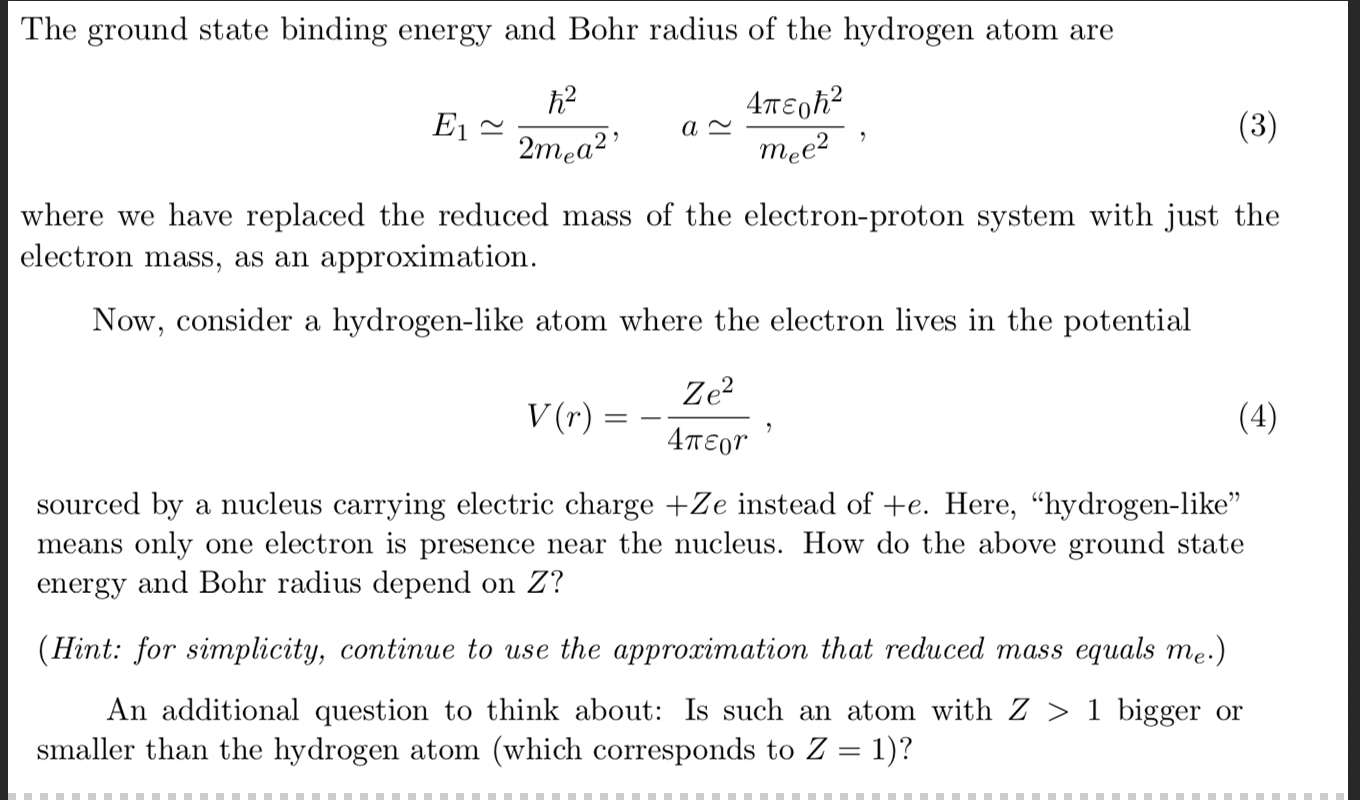
Question Video: Understanding the Effects of Electron Mass on Orbital Radius Using the Bohr Model | Nagwa